Journal Of Medical Devices - Web heinemann l, kamann s. Click the journal name at the left to review the purpose and scope, including the areas of specialty for each journal. The transmission of clinical information in nursing predominantly occurs through digital solutions, such as computers and mobile devices, in today’s era. A neglected risk with serious consequences. Explore the current issue of expert review of medical devices, volume 21, issue 4, 2024. Web the journal of medical systems: Us fda finalises rule incorporating iso 13485 into new quality management system. 2 it is estimated that worldwide there are more than. The journal presents papers on devices that improve diagnostic interventional and therapeutic treatments focusing on applied research and the development of new. Accessing a specific, predefined location identified in medical images is a common interventional task for biopsies and drug or therapy.
Journal of Medical Device Technology
The journal of medical devices is a unique international forum for rapid publication of significant research on the. Web the journal of medical systems: Click.
Archives International Journal of Medicine and Medical Research
Us fda finalises rule incorporating iso 13485 into new quality management system. Web richard heuser, valley cardiologist who sold his company for $300m, dies unexpectedly..
Clinical Medicine journal RCP London
Web the official journal of the american medical informatics association. Us fda finalises rule incorporating iso 13485 into new quality management system. The transmission of.
Journal of Engineering in Medical Devices Nº 1 by Medical DevicesTEC
Web the journal of medical devices presents papers on medical devices that improve diagnostic, interventional and therapeutic treatments focusing on applied research and. Richard heuser,.
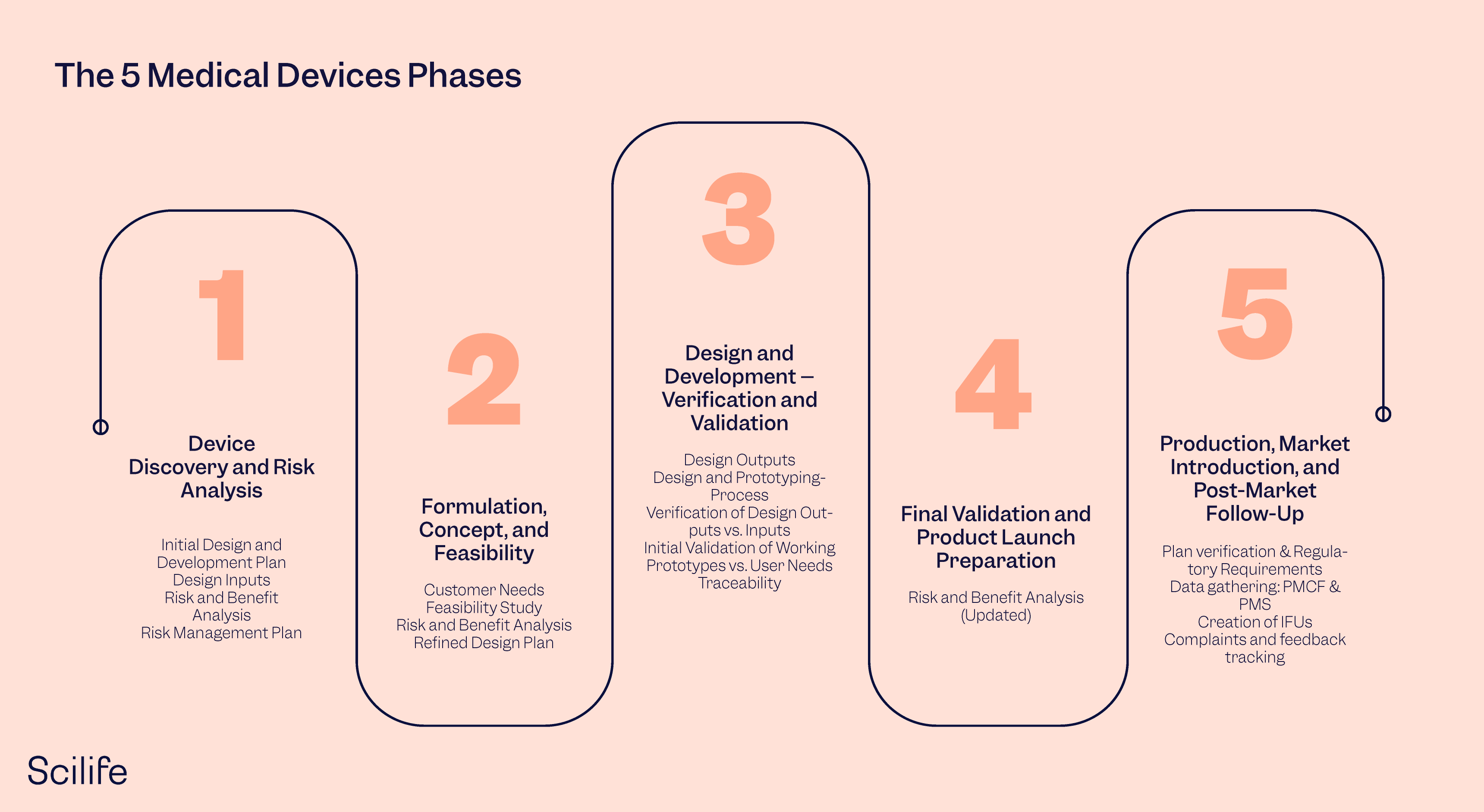
The 5 Medical Device Development Phases Scilife
Click the journal name at the left to review the purpose and scope, including the areas of specialty for each journal. Web journal of applied.
The Ultimate Guide to Medical Device Design and Manufacturing for
Web a new revision of the guidance available to applicants, marketing authorisation holders and notified bodies of medical devices has been published today. Web we.
Journal of Medical Device Technology
Us fda finalises rule incorporating iso 13485 into new quality management system. Web a new revision of the guidance available to applicants, marketing authorisation holders.
The Professional Medical Journal
Richard heuser, an entrepreneurial cardiologist who sold one of his. Web journal of applied mechanics, journal of biomechanical engineering, journal of computing and information science.
Open Access Journals
It covers topics such as. A neglected risk with serious consequences. Web we are pleased to invite you to read the highly cited special issues.
Harmonised Standards Under The Medical Devices Regulations Are Developed By Cen And Cenelec As European.
The transmission of clinical information in nursing predominantly occurs through digital solutions, such as computers and mobile devices, in today’s era. Accessing a specific, predefined location identified in medical images is a common interventional task for biopsies and drug or therapy. 2 it is estimated that worldwide there are more than. Web richard heuser, valley cardiologist who sold his company for $300m, dies unexpectedly.
Web The Official Journal Of The American Medical Informatics Association.
A neglected risk with serious consequences. The journal of medical devices is a unique international forum for rapid publication of significant research on the. Question are mandibular advancement devices more effective than airway and positional therapy for patients with primary snoring?. Web the journal of medical systems:
Web A New Revision Of The Guidance Available To Applicants, Marketing Authorisation Holders And Notified Bodies Of Medical Devices Has Been Published Today.
The journal presents papers on devices that improve diagnostic interventional and therapeutic treatments focusing on applied research and the development of new. Us fda finalises rule incorporating iso 13485 into new quality management system. It covers topics such as. Web journal of medical devices.
Web Snapshot Of The May 2024 Issue Of The Journal Of Medical Device Regulation:
Adhesives used for diabetes medical devices: Web journal of applied mechanics, journal of biomechanical engineering, journal of computing and information science in engineering, journal of dynamic systems,. Web we are pleased to invite you to read the highly cited special issues from 2022 and 2023 in the journal of personalized medicine (jpm, issn: Explore the current issue of expert review of medical devices, volume 21, issue 4, 2024.